This article is written for veterinarians, owners and trainers to summarise the major findings from a published study used for the listing of Arthramid Vet as a registered veterinary medicine in New Zealand and Australia. The full scientific article can be read here. For more information please e-mail Mail@arthramid.co.nz
Introduction
Osteoarthritis (OA) is cited as the most important musculoskeletal disorder in both humans and horses (1).
It is associated with lameness and dysfunction of the affected joint, and approximately 60% of all equine lameness is due to osteoarthritis (2). Significant economic loss to the equine industry occurs as a result and coupled with welfare concerns, motivates ongoing research into innovative treatments. The rapid resolution of lameness by a reduction in pain is paramount, but treatments ideally should also serve to arrest or slow the progression of the disease (3).
The use of an intra-articular 2.5% cross-linked polyacrylamide (2.5% PAAG- Arthramid® Vet, Contura Vet, Denmark) to treat osteoarthritis was novel. 2.5% PAAG is integrated into the synovial membrane of the joint through a combination of vessel in-growth and molecular water exchange and persists long-term in the joint (4) This significantly improves joint lameness, including that caused by both early and late stages of OA, with clinical trials showing over 82.5% of cases becoming lame-free (5, 6) for up to 12 months. Its use in flat racing Thoroughbreds is also confirmed where it was shown to significantly improve lameness and for up to 24 weeks (7).
Reason for Study- regulatory approval as a registered veterinary medicine
Intra-articular administration of 2.5% polyacrylamide hydrogel (PAAG- Arthramid® Vet) was already shown in numerous clinical trials to reduce or abolish lameness in the distal interphalangeal (coffin), metacarpo/metatarso-phalangeal (fetlock), and carpal (knee) joints of horses in both equestrian and racing disciplines. This study was the first to be performed under ‘GCP’ guidelines and was been used to meet regulatory requirements for the product to be authorised for sale to veterinarians (registered veterinary medicine).
Objective
To investigate the efficacy of 2.5% PAAG (Arthramid® Vet) in the management of inter-carpal (knee) joint lameness in racing Thoroughbreds.
Study design
Prospective double-blinded positive-control study.
Methods
33 flat-racing Thoroughbreds in full training at a single training facility with lameness (AAEP score 1-3/5) localised to the inter-carpal (middle knee) joint by intra-articular anaesthesia and radiological assessment were enrolled. Horses were randomly allocated to three treatment groups to be treated intra-articularly with either 2 ml of a 2.5% PAAG (Arthramid® Vet), 12mg of triamcinolone acetonide (TA corticosteroids) or 20mg of sodium hyaluronate (HA Hyonate) (followed by 2 further intravenous treatments of 40mg, at weekly intervals), by the treating veterinarian. All horses were rested for 48 hours post-treatment and then re-entered an unaltered training regime.
Subsequent examinations at 2, 4, and 6 weeks were performed by an examining veterinarian blinded to all treatment groups. Horses treated with 2.5% PAAG (Arthramid® Vet) were also monitored to 12 weeks for reoccurrence of lameness in the treated joint.
Results
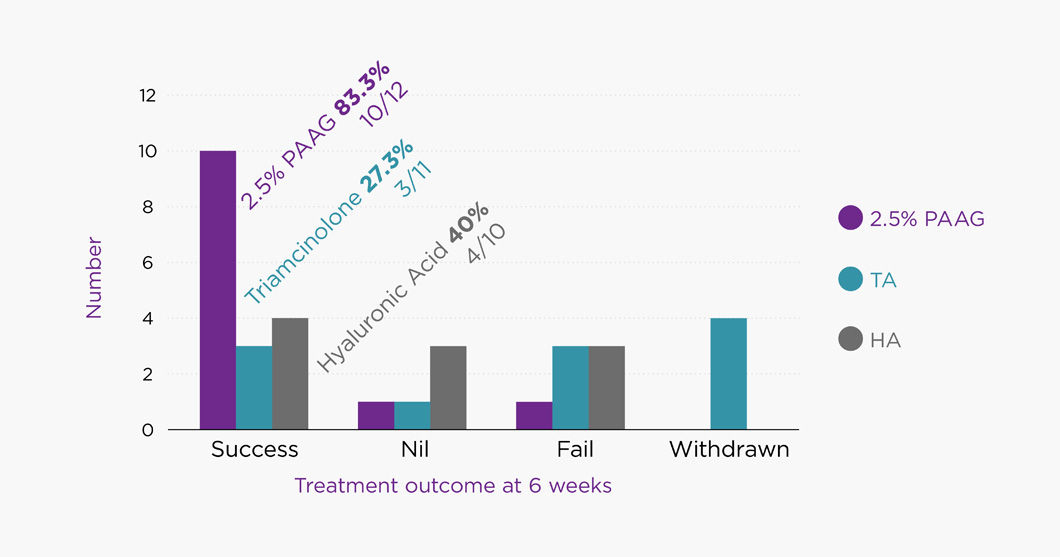
Compared to horses that received either triamcinolone acetonide or sodium hyaluronate, horses treated with 2.5% PAAG (Arthramid® Vet) showed a greater chance of resolution of lameness, joint effusion and reaction to passive flexion at four (p<0.05) and six (p<0.05) weeks. There was no difference between groups at 2 weeks. There was no significant difference between the triamcinolone acetonide and sodium hyaluronate groups at any time point. Horses treated with 2.5% PAAG (Arthramid® Vet) had a higher probability of remaining in the study (i.e. remaining in work) to 6 weeks, and 10/12 were still lame-free at 12 weeks.
Conclusions
This study was performed to Good Clinical Practice (GCP) guidelines and shows that 2.5% PAAG (Arthramid® Vet) hydrogel is both superior to and longer lasting than Triamcinolone (corticosteroids) and Hyaluronic Acid
in treating inter-carpal joint lameness in racing Thoroughbreds kept in full training. A significant reduction in joint effusion and reaction to passive flexion in the 2.5% PAAG treated group furthermore aligns with the proposed mode of action of Arthramid® Vet.
References
- van Weeren, P.R. and de Grauw, J.C., Pain in osteoarthritis. Vet Clin Equine. 2010; 26: 619-642.
- McIlwraith, C.W., Principles and practices of joint disease treatment. In: Ross, M.W., Dyson, S.J., editors. Diagnosis and management of lameness in the horse. 2nd edition. Saunders. Missouri, 2011b; 840-852.
- Caron, J.P., Intra-articular injections for joint diseases in horses. Vet Clin North Am Equine Pract. 2005; 21: 559-573.
- Christensen, L., Camitz, L., Illigen, K.E., Hansen, M., Sarvaa, R., Conaghan, P.G., Synovial incorporation of polyacrylamide hydrogel after injection into normal and osteoarthritic animal joints. Osteoarthritis Cartilage. 2016; 24: 1999-2002.
- Tnibar, A., Schougaard, H., Koene, M., Christensen, L.H., Markussen, B., A controlled clinical trial on the efficacy of an intra-articular polyacrylamide hydrogel in horses with osteoarthritis. 23rd Annual Scientific Meeting of the European College of Veterinary Surgeons (ECVS), Copenhagen, July 2014b.
- Tnibar, A., Schougaard, H., Camitz, L., Rasmussen, J., Koene, M., Jahn, W., Markussen, B., An international multi-centre prospective study on the efficacy of an intrarticular polyacrylamide hydrogel in horses with osteoarthritis: a 24 month follow up. Acta Vet Scand. 2015; 57: 20-27.
- De Clifford, L.T., Lowe, J.N., McKellar, C.D., Bolwell, C., David, F. Use of a 2.5% Cross-Linked Polyacrylamide Hydrogel in the Management of Joint Lameness in a Population of Flat Racing Thoroughbreds: A Pilot Study. Journal of Equine Veterinary Science, 2019; 77: 57-62.
Putting it into Practice
• Arthramid® Vet is superior to and longer lasting than conventional joint therapies. Understanding the complexity of disease processes associated with joint pain remains a constant challenge in clinical practice and, as with any disease process, an accurate diagnosis is essential.
• Arthramid® Vet is indicated as early as possible in the OA disease process e.g. persistent lameness abolished by intra- articular analgesia and causing synovitis or joint stiffness.
• Radiological evidence of OA is not a pre- requisite to treatment as this does not have a high correlation with clinical signs. There is anecdotal evidence for the use of AV in management of osseous cyst-like lesions.
• Arthramid® Vet can be used with other medications that may assist with subchondral bone pain. AV does not directly affect subchondral bone pain, but may aid joint function by reducing shear forces on the subchondral bone plate.
• Arthramid® Vet takes 2-4 weeks to take effect and it is not an anti-inflammatory. As a result, it requires a different mindset and owner expectations compared to conventional therapies.
Patient Management
There is evidence to suggest that a dose dependent response exists and that these doses may be altered depending on the severity or chronicity of disease.
It is important to reassess cases 4-6 weeks after first treatment. A second dose should be administered to ‘partial responders’ (< 15%) and a re-evaluation of non-responders can be undertaken.
Post-injection management can range from resting for 24 to 48hrs and then return to training, to 4 weeks of exercise restriction (e.g. swimming, water treadmill, light canter work) before return to full exercise.
Arthramid® Vet takes 2-4 weeks to integrate into the joint so better results are seen if this process is allowed to occur. As Arthramid® Vet is long-lasting it may even be prudent to consider treatment during a scheduled rest period or reduced competition demands.